Nickelback Peptide May Explain Origins of Life on Earth
Author: Rutgers University
Published: 2023/03/12 - Updated: 2025/04/11
Publication Details: Peer-Reviewed, Findings
Category Topic: Anthropology - Related Publications
Page Content: Synopsis - Introduction - Main - Insights, Updates
Synopsis: This article explores a groundbreaking scientific discovery involving a simple peptide nicknamed "Nickelback" that may have played a foundational role in the origin of life on Earth. Researchers found that this short chain of amino acids, bonded with nickel atoms, could have catalyzed essential chemical reactions billions of years ago, including the production of hydrogen - a key element in early biochemical processes. The information is compelling not only for scientists and educators but also for individuals interested in how life began, including seniors and people with disabilities who may appreciate accessible insights into complex scientific ideas. The study adds meaningful context to humanity's shared biological roots, emphasizing how even the most basic molecular structures might have influenced the evolution of life - Disabled World (DW).
- Definition: peptide
A peptide is a short chain of amino acids. The amino acids in a peptide are connected in a sequence by peptide bonds. Typically, peptides are distinguished from proteins by their shorter length, although the cut-off number of amino acids for defining a peptide and protein can be arbitrary. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular weight of 10000 or more are called proteins. Chains of fewer than twenty amino acids are called oligopeptides and include dipeptides, tripeptides, and tetrapeptides.
Introduction
Design of a Minimal di-nickel Hydrogenase Peptide - Science Advances.
A team of Rutgers scientists dedicated to pinpointing the primordial origins of metabolism - a set of core chemical reactions that first powered life on Earth - has identified part of a protein that could provide scientists clues to detecting planets on the verge of producing life.
Main Content
The research, published in Science Advances, has important implications in the search for extraterrestrial life because it gives researchers a new clue to look for, said Vikas Nanda, a researcher at the Center for Advanced Biotechnology and Medicine (CABM) at Rutgers.
Based on laboratory studies, Rutgers scientists say one of the most likely chemical candidates that kickstarted life was a simple peptide with two nickel atoms they are calling "Nickelback" not because it has anything to do with the Canadian rock band but because its backbone nitrogen atoms bond two critical nickel atoms. A peptide is a constituent of a protein made up of a few elemental building blocks known as amino acids.
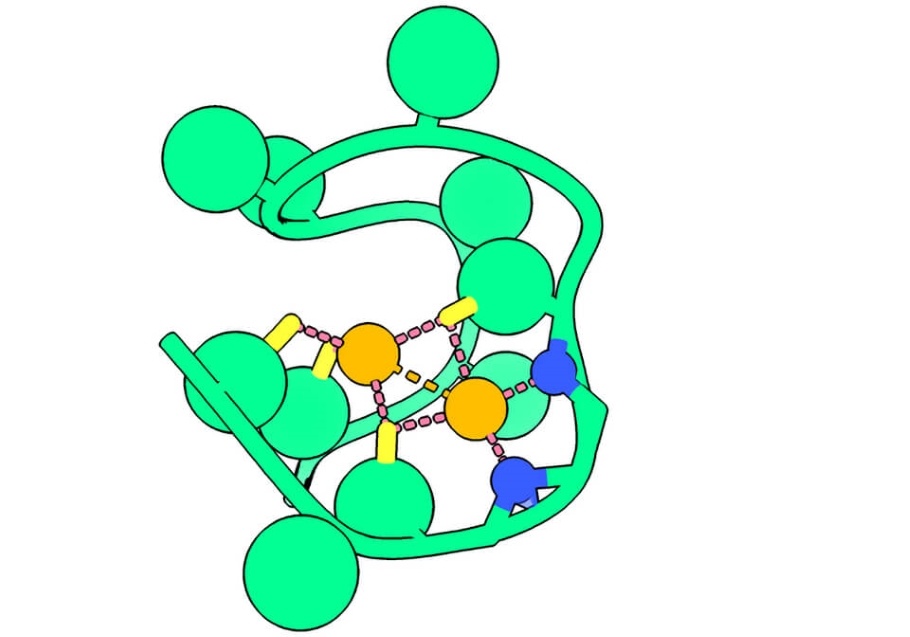
"Scientists believe that sometime between 3.5 and 3.8 billion years ago there was a tipping point, something that kickstarted the change from prebiotic chemistry - molecules before life - to living, biological systems," Nanda said. "We believe the change was sparked by a few small precursor proteins that performed key steps in an ancient metabolic reaction. And we've found one of these 'pioneer peptides.'
The scientists conducting the study are part of a Rutgers-led team called Evolution of Nanomachines in Geospheres and Microbial Ancestors (ENIGMA), part of the Astrobiology program at NASA. The researchers seek to understand how proteins evolved to become the predominant catalyst of life on Earth.
When scouring the universe with telescopes and probes for signs of past, present, or emerging life, NASA scientists look for specific "biosignatures" known as harbingers of life. Nanda said that peptides like nickelback could become the latest biosignature employed by NASA to detect planets on the verge of producing life.
An original instigating chemical, the researchers reasoned, would need to be simple enough to assemble spontaneously in a prebiotic soup. But it would have to be sufficiently chemically active to possess the potential to take energy from the environment to drive a biochemical process.
To do so, the researchers adopted a "reductionist" approach: They started by examining existing contemporary proteins known to be associated with metabolic processes. Knowing the proteins were too complex to have emerged early on, they pared them down to their basic structure.
After sequences of experiments, researchers concluded the best candidate was Nickelback. The peptide is made of 13 amino acids and binds two nickel ions.
Nickel, they reasoned, was an abundant metal in early oceans. When bound to the peptide, the nickel atoms become potent catalysts, attracting additional protons and electrons and producing hydrogen gas. The researchers reasoned that hydrogen was also more abundant on early Earth and would have been a critical energy source to power metabolism.
"This is important because, while there are many theories about the origins of life, there are very few actual laboratory tests of these ideas," Nanda said. "This work shows that not only are simple protein metabolic enzymes possible, but they are very stable and active - making them a plausible starting point for life."
Other Researchers on the Study
- Distinguished Professor Paul Falkowski and Jennifer Timm, a postdoctoral associate in the Environmental Biophysics and Molecular Ecology Program, Department of Marine and Coastal Sciences at the School of Environmental and Biological Sciences.
- Joshua Mancini, Douglas Pike, Saroj Poudel, and Alexei Tyryshkin, postdoctoral associates, and doctoral student Jan Siess at the Center for Advanced Biotechnology and Medicine and in the Department of Biochemistry and Molecular Biology at Robert Wood Johnson Medical School.
- Kate Waldie, an assistant professor of the Department of Chemistry and Chemical Biology at the School of Arts and Sciences.
- Researchers from the City College of New York also participated in the study.
COI Statement
U.S. Patent Application No. 18/047,822, "A Minimal Catalytic D-Nickel Peptide Capable of Sustained Hydrogen Evolution and Methods of Use Thereof" - current status: pending - was submitted on 19 October 2022 by Rutgers, The State University of New Jersey. Authors also listed as inventors on the patent are as follows: J.A.M., D.H.P., S.P., J.T., A.M.T., V.N., and P.G.F. The authors declare that they have no other competing interests.
Insights, Analysis, and Developments
Editorial Note: In a world where scientific understanding often feels out of reach, findings like these remind us that life's complexity may have begun with remarkable simplicity. The Nickelback peptide discovery doesn't just push the boundaries of origin-of-life research - it invites all readers, regardless of background or ability, to reflect on the extraordinary processes that shaped our existence. In a sense, it reconnects us with a universal narrative of beginnings, one that belongs to everyone - Disabled World (DW).Attribution/Source(s): This peer reviewed publication was selected for publishing by the editors of Disabled World (DW) due to its relevance to the disability community. Originally authored by Rutgers University and published on 2023/03/12, this content may have been edited for style, clarity, or brevity.