Carcinoembryonic Antigen: Clinical Applications and Controversies in Modern Oncology
Author: Ian C. Langtree - Writer/Editor for Disabled World (DW)
Published: 2025/08/10 - Updated: 2025/10/18
Publication Type: Informative
Category Topic: Journals - Papers - Related Publications
Page Content: Synopsis - Introduction - Main - Insights, Updates
Synopsis: Carcinoembryonic antigen (CEA) represents one of the most widely studied and clinically utilized tumor markers in oncology practice. Originally discovered in 1965, this glycoprotein has undergone extensive investigation regarding its diagnostic utility, prognostic significance, and therapeutic monitoring capabilities. While CEA has demonstrated considerable value in specific clinical contexts, particularly colorectal cancer management, ongoing debates persist regarding its optimal implementation, limitations, and cost-effectiveness in routine clinical practice. This comprehensive review examines the current evidence surrounding CEA, presenting both supportive and critical perspectives on its clinical utility - Disabled World (DW).
Introduction
The discovery of carcinoembryonic antigen marked a pivotal moment in the evolution of tumor marker research. Initially identified by Gold and Freedman through immunological techniques, CEA was originally thought to be uniquely expressed in embryonic tissue and malignant cells. This early understanding led to considerable optimism about its potential as a universal cancer screening tool. However, subsequent research revealed a more nuanced picture, demonstrating that CEA expression occurs in various benign conditions and that its clinical utility varies significantly across different cancer types and clinical scenarios.
CEA belongs to the immunoglobulin superfamily and functions as a cell adhesion molecule. Under normal physiological conditions, it plays roles in cell-cell adhesion and potentially in immune regulation. The protein consists of 641 amino acids and has a molecular weight of approximately 180-200 kDa, depending on the degree of glycosylation. Its structure includes an N-terminal immunoglobulin variable-like domain followed by six immunoglobulin constant-like domains.
Main Content
Historical Evolution of Discovery and Clinical Usage
The history of CEA exemplifies the arc of many diagnostic technologies: initial discovery and excitement, early adoption and overreach (screening), and subsequent refinement into targeted, evidence-based uses.
| Year | Milestone |
|---|---|
| 1965 | Initial identification of CEA by Gold and Freedman in fetal colon and colorectal carcinoma specimens. |
| Late 1960s | Early clinical studies suggested diagnostic promise for gastrointestinal cancers; interest in population screening grows. |
| 1970s | Widespread laboratory adoption and research use; screening enthusiasm peaks despite limited specificity data. |
| 1980s | Accumulating evidence of false positives from benign disease and smoking; practice shifts toward surveillance rather than screening. |
| 1990s | Assay standardization improves reproducibility; guidelines incorporate serial CEA measurement into colorectal cancer follow-up protocols. |
| 2000s | Integration with cross-sectional and functional imaging (CT, MRI, PET) enhances detection strategies; CEA examined in multi-cancer contexts. |
| 2010s | Rise of molecular diagnostics narrows the unique contribution of CEA; its role becomes more circumscribed to selected clinical uses. |
| 2020s | CEA continues as a practical surveillance tool in colorectal cancer and as part of multi-marker research panels; ongoing debate about scope of use. |
Biochemical Properties and Normal Physiology
The synthesis and regulation of CEA involve complex molecular mechanisms that remain incompletely understood. In normal tissues, CEA expression is typically restricted to certain epithelial cells, particularly those lining the gastrointestinal tract. The protein localizes primarily to the apical surface of these cells, where it may facilitate intercellular adhesion and potentially modulate immune cell interactions.
During embryonic development, CEA expression follows a distinct temporal pattern. High levels are observed during the first and second trimesters of gestation, with expression gradually declining toward term. This developmental pattern initially suggested that CEA might serve as an ideal tumor marker, given the apparent restriction of high-level expression to embryonic tissues and malignancies. However, this simplistic view was subsequently challenged by the discovery of CEA expression in various benign conditions.
The molecular mechanisms underlying CEA upregulation in malignancy involve multiple signaling pathways. Transcriptional activation appears to be mediated by various factors, including inflammatory cytokines, growth factors, and oncogenic transcription factors. Epigenetic modifications, particularly DNA methylation patterns, also influence CEA expression levels. Understanding these regulatory mechanisms has important implications for interpreting CEA levels in different clinical contexts.
Clinical Applications and Evidence
Colorectal Cancer
The most established clinical application of CEA lies in colorectal cancer management. Multiple large-scale studies have demonstrated the utility of CEA in various aspects of colorectal cancer care, though not without controversy. The evidence supporting CEA use in this context is multifaceted and requires careful examination.
For diagnostic purposes, CEA shows limited value in early-stage colorectal cancer detection. Sensitivity rates for localized disease typically range from 30-40%, making it unsuitable for primary screening. However, sensitivity increases with advanced disease, reaching 60-70% in patients with metastatic colorectal cancer. This stage-dependent sensitivity pattern reflects the relationship between tumor burden and CEA production.
The prognostic significance of preoperative CEA levels has been extensively studied. Elevated preoperative CEA correlates with advanced stage, lymph node involvement, and reduced overall survival. Several large retrospective analyses have confirmed that preoperative CEA levels provide independent prognostic information, even after controlling for traditional staging parameters. However, critics argue that this prognostic information rarely changes clinical decision-making, particularly given the availability of more sophisticated staging modalities.
Post-operative CEA monitoring represents perhaps the most controversial application of this tumor marker. Proponents argue that serial CEA measurements can detect recurrent disease earlier than conventional imaging, potentially identifying patients who might benefit from curative resection of limited metastatic disease. Several randomized controlled trials have attempted to address whether intensive CEA monitoring improves patient outcomes, with mixed results that continue to fuel debate within the oncology community.
The CEA-guided follow-up studies have generally shown that intensive monitoring leads to earlier detection of recurrence, but the critical question of whether this translates to improved survival remains contentious. Some studies suggest modest survival benefits, particularly when recurrence is amenable to surgical intervention. However, other analyses question whether these benefits justify the costs and potential psychological burden associated with intensive monitoring protocols.
Other Malignancies
Beyond colorectal cancer, CEA has been investigated in numerous other malignancies, with varying degrees of clinical utility demonstrated. In gastric cancer, elevated CEA levels correlate with advanced disease and poor prognosis, though the marker lacks sufficient sensitivity and specificity for routine diagnostic use. Similar patterns emerge in pancreatic cancer, where CEA may provide complementary information when used alongside other tumor markers like CA 19-9.
Lung cancer presents an interesting case study for CEA application. Non-small cell lung cancer, particularly adenocarcinoma, frequently demonstrates elevated CEA levels. Some investigators have explored CEA's potential role in monitoring treatment response and detecting recurrence in lung cancer patients. However, the widespread use of more specific molecular markers and the availability of advanced imaging techniques have limited CEA's role in lung cancer management.
Breast cancer represents another area where CEA has shown some clinical utility, though again with significant limitations. Elevated levels may indicate advanced disease and correlate with poor prognosis, but the marker's lack of specificity and the availability of more reliable markers like HER2 and hormone receptors have relegated CEA to a secondary role in breast cancer management.
Arguments Supporting CEA Use
Advocates for CEA utilization present several compelling arguments based on decades of clinical experience and research. The primary strength of CEA lies in its established track record and the extensive body of literature supporting its use in specific clinical contexts. Unlike newer biomarkers that may lack long-term follow-up data, CEA benefits from decades of clinical validation.
The cost-effectiveness argument represents another significant point in favor of CEA use. The assay is relatively inexpensive, widely available, and technically straightforward to perform. In healthcare systems with limited resources, CEA may provide valuable clinical information at a fraction of the cost associated with advanced imaging studies or molecular testing. This economic consideration becomes particularly relevant when considering surveillance strategies for large populations of cancer survivors.
Standardization represents another advantage of CEA testing. The assay methodology has been well-standardized across laboratories, allowing for reliable comparison of results over time and between institutions. This standardization contrasts favorably with some newer biomarkers that may suffer from inter-laboratory variability or lack of established reference ranges.
The rapid availability of results also supports CEA's clinical utility. Unlike tissue-based molecular testing that may require days or weeks for completion, CEA results are typically available within hours. This rapid turnaround time can be particularly valuable in clinical situations requiring prompt decision-making.
Proponents also emphasize CEA's role in treatment monitoring. Serial measurements can provide objective evidence of treatment response or disease progression, potentially guiding therapeutic decisions. While imaging remains the gold standard for response assessment, CEA may provide complementary information, particularly in situations where imaging findings are ambiguous or when frequent imaging would be impractical.
Arguments Against CEA Use
Critics of routine CEA use present equally compelling arguments that challenge its clinical utility and cost-effectiveness. The fundamental limitation of CEA lies in its lack of specificity, both for malignancy in general and for specific cancer types. Elevated CEA levels can occur in numerous benign conditions, including inflammatory bowel disease, liver disease, smoking, and various infections. This lack of specificity leads to false-positive results that may cause unnecessary anxiety and prompt expensive additional testing.
The sensitivity limitations of CEA represent another significant concern. Many early-stage cancers do not produce elevated CEA levels, potentially providing false reassurance and delaying appropriate diagnostic workup. This sensitivity limitation is particularly problematic when considering CEA for screening purposes, where early detection is the primary goal.
The psychological impact of CEA monitoring deserves serious consideration. Patients undergoing regular CEA surveillance may experience significant anxiety, particularly when levels show minor fluctuations that may be clinically insignificant. This psychological burden must be weighed against any potential clinical benefits, particularly given the uncertain survival advantages associated with intensive monitoring protocols.
Cost-effectiveness analyses have produced mixed results regarding CEA's economic value. While the individual test cost is relatively low, the cumulative costs associated with routine surveillance, including follow-up testing for elevated results, can be substantial. Some health economic analyses suggest that resources might be better allocated to other aspects of cancer care that demonstrate clearer survival benefits.
The availability of superior alternatives also challenges CEA's continued use in many clinical contexts. Advanced imaging techniques, molecular markers, and liquid biopsy technologies may provide more accurate and clinically relevant information than CEA in many situations. As these technologies become more accessible and cost-effective, the relative value of CEA may continue to decline.
Technical Considerations and Limitations
The technical aspects of CEA testing introduce several important considerations that influence its clinical utility. Assay methodology has evolved significantly since the marker's initial discovery, with modern immunoassays providing improved precision and reproducibility compared to earlier techniques. However, different assay platforms may produce varying results, necessitating careful attention to the specific methodology used and appropriate reference ranges.
Biological variation represents another important technical consideration. CEA levels can fluctuate in response to various physiological factors, including smoking status, renal function, and inflammatory conditions. Heavy smokers typically demonstrate elevated baseline CEA levels, requiring adjusted interpretation of results. Similarly, patients with impaired renal function may show elevated CEA levels due to reduced clearance rather than malignancy.
The timing of CEA measurements relative to treatment interventions can significantly influence results interpretation. Post-operative CEA levels may remain elevated for several weeks following surgical resection, reflecting the time required for clearance of circulating antigen. Similarly, chemotherapy may cause transient CEA elevations due to tumor cell destruction, a phenomenon that must be distinguished from disease progression.
Quality control measures are essential for reliable CEA testing. Laboratories must implement appropriate internal quality control procedures and participate in external proficiency testing programs to ensure accurate and reproducible results. The stability of CEA in different sample types and storage conditions also requires careful attention to pre-analytical variables.
Comparative Summary of Strengths & Weaknesses
| Aspect | Strengths | Weaknesses |
|---|---|---|
| Cost & Accessibility | Low-cost, widely available in clinical labs. | Poor specificity prevents justified use in population screening. |
| Monitoring Recurrence | Good for longitudinal tracking in selected cohorts (e.g., postoperative colorectal patients). | Not all tumors secrete CEA; low-volume disease may be missed. |
| Diagnostic Role | Useful adjunct to imaging and pathology in known cancer cases. | Cannot reliably distinguish benign from malignant causes of elevation. |
| Prognostic Value | Pre-op levels correlate with stage and outcomes in colorectal cancer. | Prognostic utility is less consistent outside colorectal malignancies. |
| Patient Impact | Stable or falling values can reassure patients and clinicians. | False alarms cause anxiety and can drive overtreatment. |
NOTE: Clinical interpretation of CEA requires context: smoking status, liver function, concurrent inflammatory disease, and the specific cancer histology must all be considered before acting on a numeric value.
Current Guidelines and Recommendations
Professional organizations have developed various guidelines addressing CEA use in clinical practice, though recommendations differ somewhat between organizations and continue to evolve as new evidence emerges. The American Society of Clinical Oncology has issued specific guidelines regarding tumor marker use, including CEA, that attempt to balance the available evidence with practical clinical considerations.
For colorectal cancer, most guidelines support the use of preoperative CEA measurement for prognostic purposes, though the clinical utility of this information remains debated. Post-operative surveillance recommendations vary more significantly between organizations, with some supporting routine CEA monitoring and others recommending more selective use based on individual patient characteristics.
The European Society for Medical Oncology has issued similar guidelines that generally align with American recommendations but emphasize the importance of individualizing surveillance strategies based on patient factors such as age, comorbidities, and treatment history. These guidelines acknowledge the ongoing controversies surrounding CEA use while attempting to provide practical guidance for clinicians.
International guidelines increasingly emphasize the importance of multidisciplinary decision-making regarding tumor marker use. The decision to implement CEA monitoring should involve input from surgical, medical, and radiation oncologists, as well as consideration of patient preferences and quality of life factors.
Future Directions and Emerging Technologies
The future role of CEA in clinical practice will likely be influenced by several emerging trends and technologies. Liquid biopsy techniques, including circulating tumor DNA analysis, may provide more specific and sensitive markers of disease recurrence than traditional protein-based markers like CEA. However, the clinical utility and cost-effectiveness of these newer technologies remain under investigation.
Artificial intelligence and machine learning approaches may enhance the interpretation of CEA results by integrating multiple biomarkers and clinical variables to provide more accurate risk stratification. These computational approaches might help address some of the limitations associated with CEA's lack of specificity by providing more sophisticated interpretive frameworks.
Point-of-care testing technologies may increase the accessibility and convenience of CEA testing, particularly in resource-limited settings. However, the clinical utility of more frequent or convenient testing remains to be established, and concerns about over-testing and false-positive results may actually increase with improved accessibility.
The development of CEA-targeted therapeutic approaches represents another potential future direction. CEA-targeted immunotherapies and antibody-drug conjugates are under investigation, potentially providing new treatment options for CEA-expressing tumors. If these approaches prove clinically successful, the role of CEA testing might expand beyond monitoring to include therapeutic selection.
Economic Considerations
The economic impact of CEA testing extends beyond the direct costs of laboratory analysis to include downstream effects such as additional imaging, specialist consultations, and psychological support services. Comprehensive economic analyses must consider these broader cost implications when evaluating CEA's overall value proposition.
Healthcare resource allocation decisions increasingly require rigorous cost-effectiveness analyses that compare different surveillance strategies. CEA-based monitoring protocols must demonstrate not only clinical efficacy but also economic efficiency when compared to alternative approaches such as imaging-based surveillance or clinical follow-up alone.
The economic burden of false-positive CEA results deserves particular attention. Each false-positive result may trigger expensive diagnostic workups, including advanced imaging studies and potentially invasive procedures. The cumulative cost of these false-positive evaluations can be substantial and must be weighed against the benefits associated with true-positive results.
International variations in healthcare systems and resource availability influence the economic attractiveness of CEA testing. In settings where advanced imaging is readily available and affordable, the relative value of CEA monitoring may be lower than in resource-limited environments where CEA provides an accessible alternative to more expensive surveillance modalities.
Patient Perspectives and Quality of Life
The patient experience with CEA monitoring involves complex psychological and quality-of-life considerations that are often overlooked in purely clinical analyses. For many cancer survivors, regular CEA testing provides reassurance and a sense of active participation in their ongoing care. This psychological benefit may justify continued monitoring even in the absence of clear survival advantages.
Conversely, some patients experience significant anxiety related to CEA testing, particularly when results show minor elevations or fluctuations. The emotional impact of "watching the numbers" can be substantial, potentially diminishing quality of life during the survivorship period. Healthcare providers must carefully assess individual patient preferences and coping mechanisms when making recommendations about CEA monitoring.
The communication of CEA results requires particular sensitivity and skill. Patients need to understand the limitations of the test, including the possibility of false-positive and false-negative results. This educational component is essential for informed decision-making and appropriate expectations regarding test performance.
Cultural factors may also influence patient attitudes toward CEA testing. Some cultures may place greater emphasis on aggressive monitoring and early detection, while others may prioritize quality of life over intensive surveillance. These cultural considerations should inform individualized recommendations regarding CEA use.
CEA Reference Ranges and Clinical Interpretation
| Patient Population | CEA Level (ng/mL) | Clinical Significance | Recommended Action |
|---|---|---|---|
| Healthy Non-smokers | <2.5 | Normal range | Routine follow-up |
| Healthy Smokers | <5.0 | Elevated due to smoking | Consider smoking cessation counseling |
| Benign Conditions | 2.5-10.0 | May indicate inflammatory process | Investigate underlying cause |
| Malignancy Suspected | >10.0 | Concerning for malignancy | Urgent oncological evaluation |
| Post-operative Baseline | Variable | Establishes individual baseline | Serial monitoring recommended |
| Rising Trend (>25% increase) | Any level | Possible disease progression | Consider imaging and clinical assessment |
| Markedly Elevated | >20.0 | High probability of advanced disease | Immediate comprehensive evaluation |
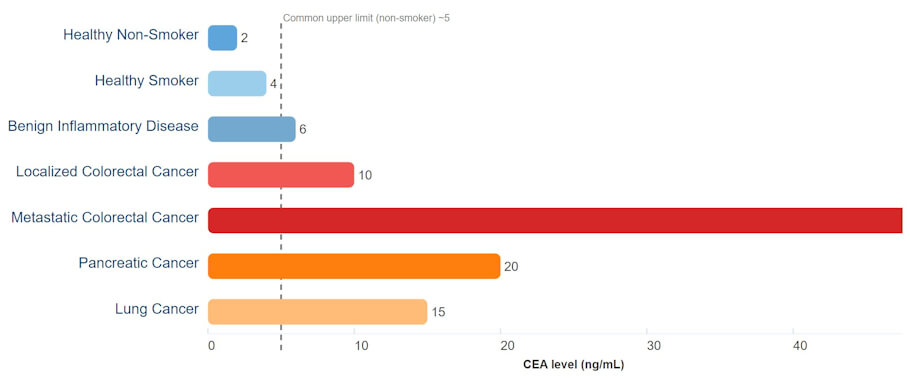
Infographic: Typical CEA Levels by Condition
Representative CEA values (ng/mL). These are illustrative averages, not diagnostic cut-offs; overlap between benign and malignant conditions is common.
NOTE: The infographic-style chart above shows representative CEA levels across various benign and malignant conditions, with a dashed line marking the common clinical upper limit for non-smokers. This makes it visually clear why CEA alone can’t reliably distinguish cancer from other causes of elevation.
Regional and Institutional Variations
Clinical practice regarding CEA use varies significantly between different regions and institutions, reflecting ongoing uncertainties about optimal implementation strategies. Academic medical centers may be more likely to implement intensive monitoring protocols, while community practices might adopt more conservative approaches based on resource constraints and patient populations.
International differences in CEA utilization patterns reflect varying healthcare system structures, reimbursement policies, and clinical traditions. European practices may emphasize different aspects of CEA testing compared to North American approaches, with these variations providing opportunities for comparative effectiveness research.
Institutional policies regarding CEA use often reflect local expertise and resources. Centers with strong surgical programs might emphasize CEA's role in identifying patients suitable for metastasectomy, while institutions with limited surgical resources might focus on prognostic applications.
Conclusion
Carcinoembryonic antigen remains a clinically relevant but controversial tumor marker more than five decades after its discovery. The evidence supporting its use in specific clinical contexts, particularly colorectal cancer management, is substantial but not without limitations. The marker's lack of specificity, variable sensitivity, and uncertain impact on patient outcomes continue to generate debate within the oncology community.
The decision to implement CEA testing should be individualized based on multiple factors, including cancer type, stage, patient preferences, available resources, and institutional expertise. While CEA may provide valuable information in carefully selected situations, routine use without clear clinical indications appears increasingly difficult to justify given the availability of alternative approaches and the ongoing questions regarding cost-effectiveness.
Future research should focus on identifying patient subgroups most likely to benefit from CEA monitoring, developing more sophisticated interpretive algorithms, and conducting rigorous comparative effectiveness studies. The integration of CEA with newer biomarkers and advanced imaging techniques may ultimately provide more effective surveillance strategies than any single approach alone.
As precision medicine continues to evolve, the role of traditional tumor markers like CEA will likely become more targeted and specific. Rather than broad application across all patients with a particular cancer type, CEA use may become increasingly refined based on molecular characteristics, risk stratification, and individual patient factors. This evolution toward personalized monitoring strategies represents the most promising path forward for optimizing CEA's clinical utility while minimizing its limitations and costs.
Printable Carcinoembryonic Antigen Chart
NOTE: Prepared as an educational clinical review. This document does not provide medical advice — clinicians should integrate CEA results with clinical findings, imaging, and pathology, and follow local guidelines.
Insights, Analysis, and Developments
Editorial Note: The ongoing debate surrounding CEA underscores a broader challenge in modern oncology: distinguishing between diagnostic capability and clinical benefit. While technology enables us to measure more, measure faster, and detect changes more frequently, these capabilities do not automatically improve patient outcomes. The most valuable surveillance strategy recognizes that cancer monitoring serves patients best when it balances realistic prospects for improved treatment with genuine quality of life considerations—a balance that demands individualizing recommendations rather than applying uniform protocols to all. As the field moves toward precision medicine, CEA's future likely lies not in universal application but in targeted use for carefully selected patient populations where the marker's limitations are minimized and its clinical value demonstrated, reminding us that the most sophisticated oncology ultimately remains that which aligns technology with genuine patient benefit - Disabled World (DW). Author Credentials: Ian is the founder and Editor-in-Chief of Disabled World, a leading resource for news and information on disability issues. With a global perspective shaped by years of travel and lived experience, Ian is a committed proponent of the Social Model of Disability-a transformative framework developed by disabled activists in the 1970s that emphasizes dismantling societal barriers rather than focusing solely on individual impairments. His work reflects a deep commitment to disability rights, accessibility, and social inclusion. To learn more about Ian's background, expertise, and accomplishments, visit his full biography.
Author Credentials: Ian is the founder and Editor-in-Chief of Disabled World, a leading resource for news and information on disability issues. With a global perspective shaped by years of travel and lived experience, Ian is a committed proponent of the Social Model of Disability-a transformative framework developed by disabled activists in the 1970s that emphasizes dismantling societal barriers rather than focusing solely on individual impairments. His work reflects a deep commitment to disability rights, accessibility, and social inclusion. To learn more about Ian's background, expertise, and accomplishments, visit his full biography.